Solution
The reaction is an endothermic
reaction. Heat is absorbed as the temperature decreases.
The calibration factor of the calorimeter is given by the expression below.
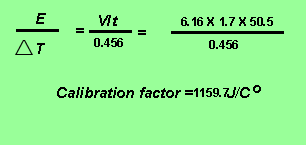
Obtain the amount of energy absorbed from 1.01 grams of potassium nitrate
![]()
Calibration factor X change in temperature = 1159.7 X 0.672 = 0.779KJ
The enthalpy change is energy/mol
SO!
Step 1) Mole of potassium nitrate added = 1.01 /101 = 0.01 mole
Step 2) Divide the amount of energy by the number of mole of potassium nitrate.
0.779 / 0.01 = 77.9Kj/mol
Note how the enthalpy change has a positive sign. This indicates an endothermic reaction (heat absorbed from the surroundings).
Click to hide the solution