Purifying iron -blast furnace |
||
Removing the oxygen from iron oxide (iron ore) takes a great deal of energy. The process must be done on a large scale and be relatively cheap. This is a very important industry for a developed nation and can be quite polluting as any picture of a steel works will reveal. Removing the oxygen involves a chemical reaction that gives off a great deal of heat energy. Click to see a demonstration of oxygen being removed from rust, by aluminium, to form pure iron. This method of removing oxygen from the iron is not possible on a large scale as the aluminium powder is too expensive. |
|
|
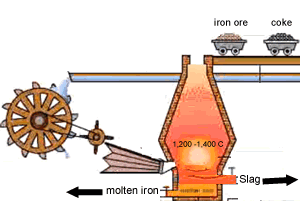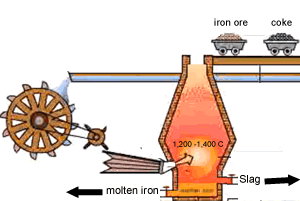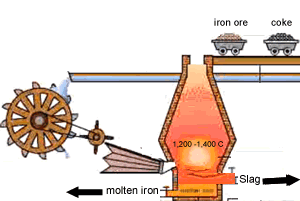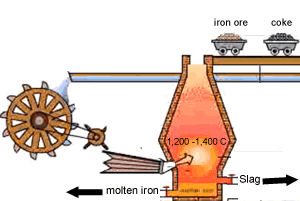
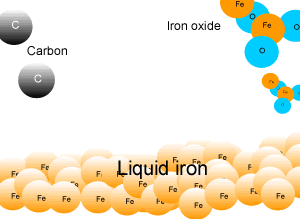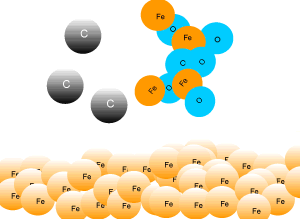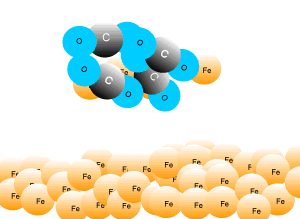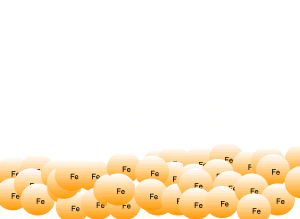
A blast furnace is used in industry to convert iron oxide to iron. A blast furnace is a tall chimney like structure, lined with heat resistant bricks. Iron ore and carbon are placed in the blast furnace at the top. Limestone is also added to remove silica which is present as sand and stone. This forms a substance called slag that floats on top of the molten iron. Slag is poured off, collected and removed. The simple animation on the right shows how the blast furnace works. Air is pumped at the bottom, not exactly as shown in the diagram. As the air rises it heats up and reacts with the coal that is constantly fed in at the top. The blast furnace operates 24 hours a day and is only shut down when repairs have to be made.
|
|
|
In industry, carbon is used to remove the oxygen from the iron oxide. A simple animation is shown on the right. In junior science it is sufficient to appreciate that carbon takes the oxygen from the iron oxide leaving pure iron to sink to the bottom of the blast furnace. |
|
|
|
||
1) Will sand melt at temperatures below 1,400 C?
|
||
2) Why does slag float on top of the molten iron?
|
||
3) What two criteria are important for iron to be used widely in construction?
|
||
4) In simple terms, what is the purpose of adding carbon to iron oxide?
|
||
5) What is a major gas pollutant produced by the furnace?
|
||
6) The carbon that is fed in at the top of the furnace serves to:
|
||
Continue with the chemistry of the blast furnace. |
||