
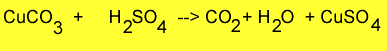
When an acid is added to a carbonate water, carbon dioxide and a salt are formed. We can identify the gas by placing a burning match in the beaker where the acid and the carbonate react. Click to see a 120kb video and identify the gas. What are two properties of carbon dioxide that are used to identify it in the video.
Step 1 Weigh approximately 1 gram of copper carbonate and place it into a beaker. Record the mass accurately.
Step 2 Add 5mls of 1.0M sulfuric acid and observe the mixture fiz. When the fizzing stops add 1ml of sulfuric acid. Continue to add 1ml of sulfuric acid until no more fizzing occurs.
Step 3 The solution should look blue as in the picture on the right. This is due to the formation of copper sulfate.

