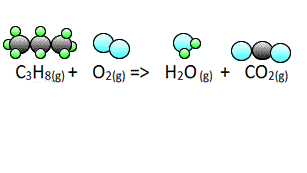Now lets see how we derive the coefficients for the balanced equation when
propane gas reacts with oxygen to form carbon dioxide gas and water vapour.
To start balancing the equation it is easier to pick an element that appears once on each side of the equation. In this case you can start with either hydogen or carbon
as oxygen appears twic on the right of the equation
Step 1 Let's start with carbon. We have three carbon atoms on the left and one on the right so
lets put three molecules of CO2 on the right. This will balance for carbon.
Step 2 Now let's balance for hydrogens. We have eight hydrogen atoms on the left and two on the right, so lets put four H2O molecules to the right side to make a total of 8 hydrogen atoms.
Step 3 Now we have to balance for oxygen atoms. We have 2 atoms on the left and ten atoms on the right, so let's put five molecules of oxygen on the left