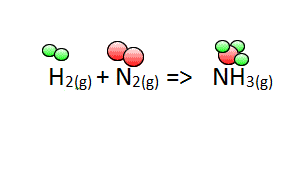Now lets see how we derive the coefficients for the balanced equation when
Hydrogen gas(H2) reacts with nitrogen gas(N2) to form ammonia gas (NH3)
To start balancing the equation it is easier to pick an element that appears once on each side of the equation. In this case you can start with either nitrogen or hydrogen
Step 1 Let's start with nitrogen. We have two nitrogen atoms on the left and one on the right so
lets put two molecules of NH3 on the right. This will balance for nitrogen.
Step 2 Now let's balance for hydrogens. We have six hydrogen atoms on the right and two on the left, so lets add another 2 H2 molecules to the left side to make a total of 3 hydrogen molecules.