Polymers and some of their special properties.
One common polymer used to make hydrogels is sodium polyacrylate.
The chemical name for this is poly(sodium propenoate).
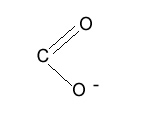
Sodium polyacrylate is a long, randomly coiled polymer.
If you remove all the the salt (Na+) the negative charges on the oxide ions along the polymer chain all repel each other and the chains tend to uncoil. Water molecules are attracted to the negative charges by hydrogen bonding. In this state the hydrogel can absorb over five hundred times
its own weight of pure water but less if the water is salty.
This ability to absorb so much water makes the hydrogel useful for the lining of baby nappies.
When salt is added to the hydrogel, the chains
start to change their shape and water is lost from the gel
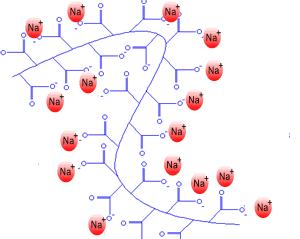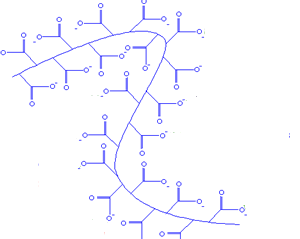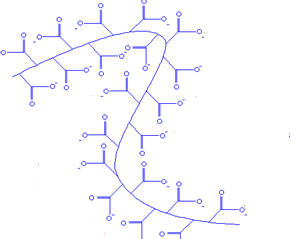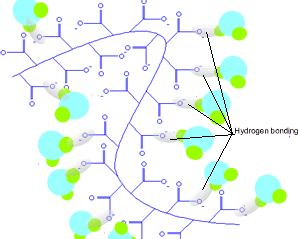



The polymer swells as it absorbs the water to form a thick gel.
