Fireworks in a test tube
You will need :
- Conc. sulfuric acid
- Methanol
- KMnO3
- spatula
- small test tube
- Test tube rack
A spectacular display of fireworks occurs when concentrated sulfuric acid and methanol are placed in a test tube.
Caution - concentrated sulfuric acid is extremely corrosive. Gloves and goggles must be worn.

The reaction can get very hot and spill over the test tube. Best to do this over a sink or in a plastic bucket.
Step 1 Place 2 mL of sulfuric acid into a test tube.
Step 2 Carefully, using an eye-dropper, add about 5 mL of methanol. Avoid any mixing of the two liquids. The demonstration won't work if the two liquids are mixed. Do not handle the test tube beyond this point.
Step 3 Add a few crystals of KMnO3 and turn the light out. Ideally this should be done in a fume cupboard.
Which of the following is an indication that a chemical reaction is taking place?
A strong oxidant is used in this reaction. An oxidant is
An organic compound is oxidised in this reaction. Organic compounds are composed of hydrogen, carbon and sometimes oxygen. Which one of the following chemicals is an organic compound?
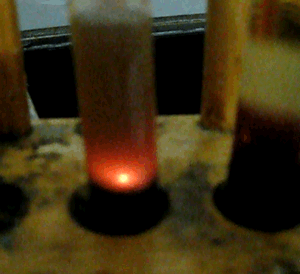
View the video on the right of the reaction.
The chemistry behind the reaction
In the presence of sulfuric acid, MnO4- is converted to Mn2O7, manganese heptoxide, according to the equation
- 2 KMnO4 + 2 H2SO4 → Mn2O7 + H2O + 2 KHSO4
Manganese heptoxide is a powerful oxidant that oxidizes organic molecules with the strong evolution of heat.
Questions for senior Chemistry.
View the video on the right. Manganese heptoxide is oxidizing sugar and is itself being reduced to manganese dioxide (MnO2) and oxygen gas
Write the half equation for the reduction of manganese heptoxide.
What is the oxidation states of Mn in Mn2O7 and MnO2?
Write the half equation for the complete oxidation of sugar (C6H12O6) to water and carbon dioxide.
Write the overall equation for the oxidation of sugar by manganese heptoxide.
What is the oxidation sate of carbon before and after oxidation of sugar?