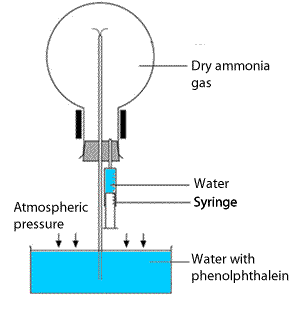
This demonstration experiment can be used to illustrate the very high solubility of ammonia in water. Ammonia forms an alkaline solution when dissolved in water according to the equation below.
NH3(aq) + H2O(l) => NH4+(aq) + OH-(aq).
You will need
Eye protection
Access to a fume cupboard
Flask, 500 mL or 1L which is completely dry
Rubber stopper, plain, to fit the flask
Two-holed rubber stopper to fit the flask fitted with a glass jet
Plastic syringe (10 mL)
Trough or large beaker which can hold more water than the flask
Stand, clamp and boss along with a heavy weight or bench clamp to avoid apparatus toppling over
White board or sheet of card to act as a background to improve visibility
Acid-alkali indicator (e.g.phenolphthalein)
Access to a fume cupboard
Stand, clamp and boss
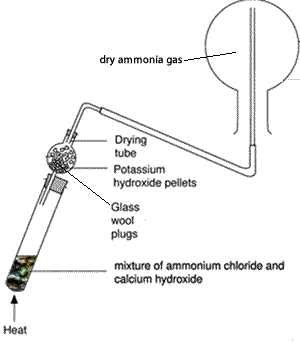
Boiling-tube fitted with a one-holed rubber stopper holding a drying tube.
One-holed rubber stopper to fit the drying tube, fitted with a short length of glass delivery tube
Length of rubber delivery tube
Bunsen burner
Heat resistant mat
Ammonium chloride, 5 g
Calcium hydroxide , 5 g
Set up the apparatus as shown on the right. Using a syringe inject a tiny amount of water in the now sealed flask. The pressure in the flask drops as the ammonia gas quickly dissolves in the water.
A column of water is pushed up the tube from the water reservoir into the flask. As the phenolphthalein enters the flask it turns pink as it reacts with the alkaline water inside the flask.