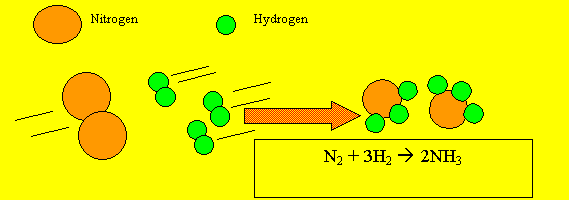
Scientists use a special way of writing to describe what happens in a chemical reaction. They describe a chemical reaction by writing a chemical equation using chemical symbols to represent the atoms taking part. Below is an example of a chemical equation using chemical formulae instead of words..
![]()
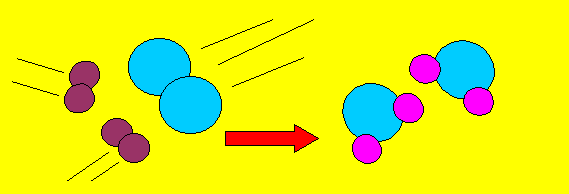
This equation
tells us that two molecules of hydrogen gas react with one molecule
of oxygen gas to produce two molecules of water. The equation has two
sides. On the left of the arrow we put the reactants(the chemicals that
react together). On the right side of the arrow we put the products
(the chemicals) that are formed or produced.
If we were to draw this reaction in pictures it would look like this.
Why do we have
a 2 in front of the water and the hydrogen molecule in the equation?
This is called "BALANCING the equation".
There is a special Law in Nature that states that "Matter can
not be created or destroyed". It is called "The Law Of
Conservation Of Mass".
Click to see a demonstration of this
law.
What this means
is that we have to have the same number of each atom on each side of
the arrow in the equation.
Methane and oxygen react to form carbon dioxide and water
CH4 + 2O2 => CO2 + 2H2O
The equation reveals that molecule/s of methane react/s with molecule/s of oxygen to form molecule/s of carbon dioxide and molecule/s of water.
How many oxygen atoms on the right of the equation
How many oxygen atoms on the left of the equation
How many hydrogen atoms on the right of the equation
How many hydrogen atoms on the left of the equation
How many carbon atoms on the right of the equation
How many carbon atoms on the leftt of the equation

Write an equation for each of the reactions on the next page.
Here is an example