Intra-molecular bonding
Intra-molecular bonding refers to the bonding within a molecule.
Two types of covalent bonds exist within molecules:
- polar covalent;
- pure covalent.
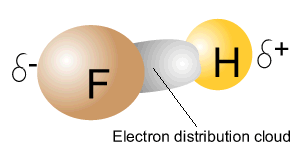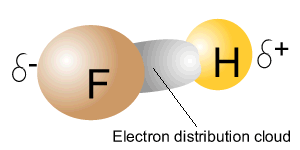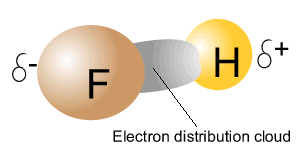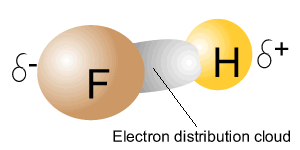
The image on the right shows a molecule of FH . Due to a difference in electronegativity between fluorine and hydrogen, electrons are unevenly shared between the two atoms. This causes the covalent bond to become polar. The side of the covalent bond where electrons spend more time (dark grey area pictured int he animation) will have a slight positive charge while the other side of the bond has a slight positive charge. This type of covalent bond is known as a polar-covalent bond and occurs between two atoms with different electronegativity.
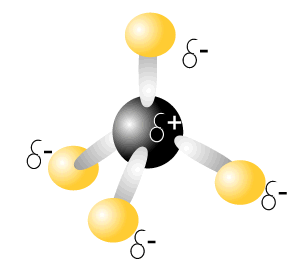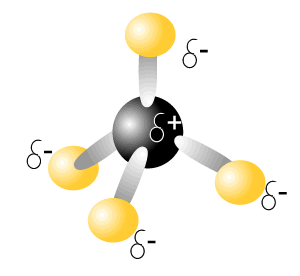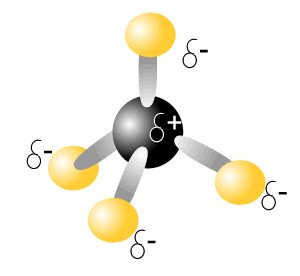
Consider the molecule of carbon tetrachloride (CCl4), on the right. This molecule has 4 polar covalent bonds.
So we say that the intramolecular bonding of this is polar-covalent.
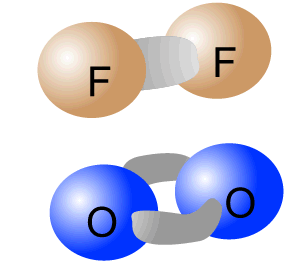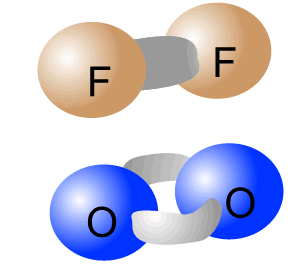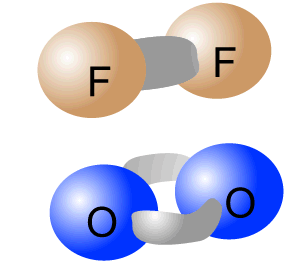
Pure-covalent bonds occur between atoms with identical electronegativity. In this way the electrons are evenly shared between the two atoms forming the bond.
The intramolecular bonding of F2 and O2 is pure-covalent.