Graphite
Covalent
bonding in 2 dimensions
Graphite and diamond are allotropes of carbon. They are different structural forms of the same element, carbon. Graphite's structure is very different to that of diamond. Carbons are bonded to three other carbon atoms in the same layer to form a six member ring. The rings of carbon form layers, as pictured on the right, that are very strongly bonded in 2 dimensions by covalent bonds. The bonding between the layers is very weak attributed to weak dispersion forces only. This causes the layers to slide past one another with relative ease and is the reason why graphite is used as a lubricant for heavy machinery.
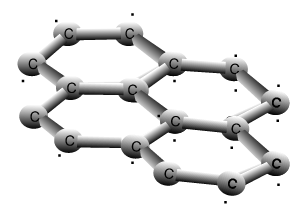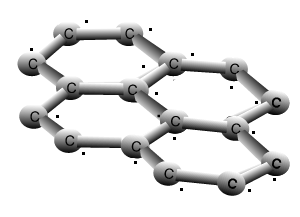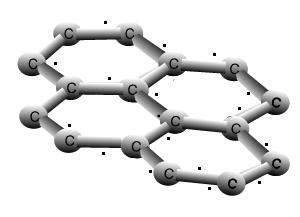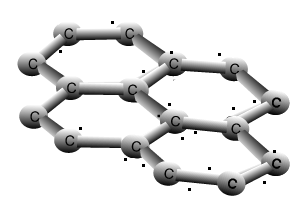
Graphite
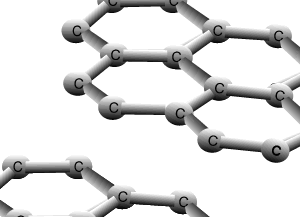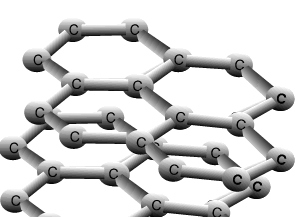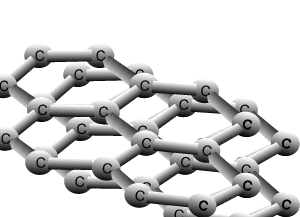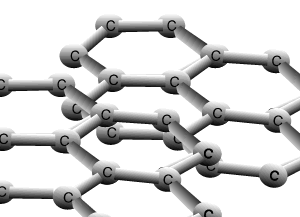
The animation above shows the layers of graphite sliding past one another with relative ease. Weak dispersion forces only act between the layers of graphite, while the fourth valence electron from each carbon is delocalised.
Each carbon atom in graphite
is bonded to three others. The double bond forms a circular pattern as it relocates around the 6 membered ring. This
causes one of the bonding electrons to become delocalised and move freely along the layers.
Delocalised electrons give graphite the ability to conduct electricity
in the solid state.