Atoms reacting continued
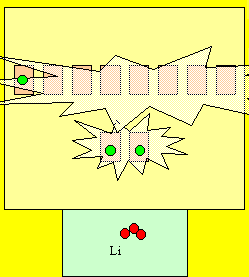
Lets continue with lithium. Lithium has 3 electrons. So we always fill the bottom levels first. It takes less energy,
as you well know when you climb stairs. So, two electrons fit snugly in the first level and the third must go up to the second level. The second level is energised for one electron. Is this energy efficient? Click to see how
reactive lithium can be.
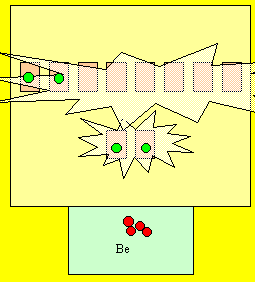
Berylium has 4 electrons and looks like the
diagram on the right. Is this 100%
energy efficient?
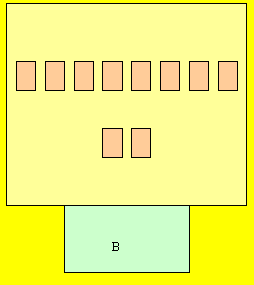
Complete the rest of the atoms on
your own. Don't forget the protons
in the basement (nucleus).