1) The following are the electronic configurations of the elements below
a) Element X
1s2, 2s2, 2p6, 3s2, 3p6,
3d10, 4s2, 4p6
b) Element Y 1s2, 2s2, 2p6, 3s2,
3p6, 3d10, 4s2, 4p4
c) Element Z 1s2, 2s2, 2p6, 3s1
d) Element X 1s2, 2s2, 2p6, 3s2,
3p6, 3d10, 4s2, 4p5
What group and period will each element be found?
Which element is a noble gas?
Which element is considered
to be an alkali metal?
Which element has the greatest electronegativity?
Which element has the lowest first ionisation energy?
Which element is a halogen?
Which element is the weakest oxidant?
2) The graphs below show the trends of some properties of atoms as we travel down group 2. Match each of the following properties with one of the graphs.
a) Oxidation state of the
metal ion
b) First ionisation energy
c) Atomic radius
d) Electronegativity
e) Oxidising strength
f) Reducing strength
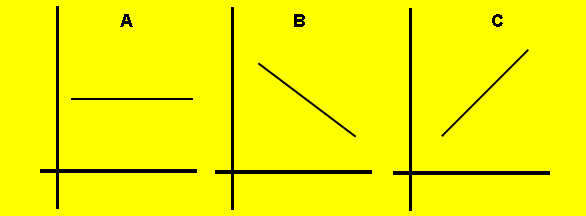
2) The graphs above show the trends of some properties of atoms as we travel across period 2 from lithium to neon. Match each of the following properties with one of the graphs.
a) First ionisation energy
b) Atomic radius
c) Electronegativity
d) Oxidising strength
e) Reducing strength
3) Across period 3, the properties of the oxides of each element change. Explain how the properties change with reference to relevant equations.