Complex ions
Stability constants
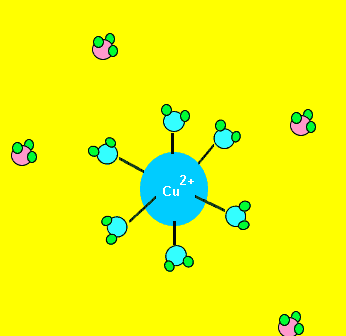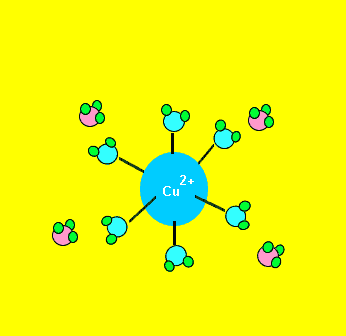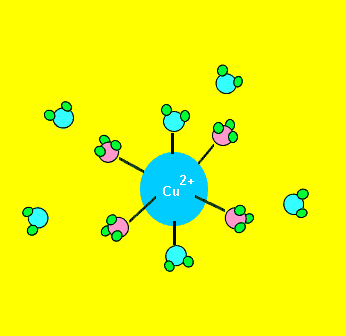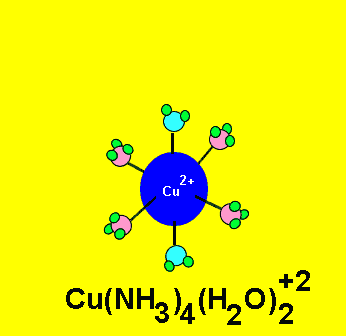
Some complex ions are more stable than others. Certain ligands can replace existing ligands, as shown on the left. For example, the water molecules from the Cu(H2O)62+ complex ion can be replaced by NH3 molecules. The Cu(NH3)42+ is more stable and produces a deep blue colour.
Cu2+(aq) + 4NH3(aq) <=> Cu(NH3)4+2(aq)
The equilibrium expression for the above reaction can be written as
Kst = [Cu(NH3)4+2]
/ ([Cu2+][NH3]4) = 1012 at
25oC
Kst is also known as the stability constant.
The equilibrium constant, at 1012 , is very high and this equilibrium
will take place in preference to the formation of the Cu(H2O)62+
complex ion.
How to use stability constants.
SIlver ions(Ag+)
are placed in a solution containing 0.1M NaCl and 0.1M NH3
. Assuming we know the stability constants of the following equilibria
at some unknown temperature, what will be the most abundant complex
ion in the mixture?
Ag+(aq) + 2NH3+(aq) <=> Ag(NH3)2+(aq)
Kst = 107
Ag+(aq) + 2Cl-(aq) <=> Ag(Cl)2-(aq) Kst = 10
Looking at the stability constants we see that the formation of Ag(NH3)2+ is favored. The dominant complex will be Ag(NH3)2+.