Transition metal revision
Sketch the complex
ion Fe(H2O)63+
Label the bonds:
- between the Fe3+ ion and the H2O molecule
- within the H2O molecule.
Suggest a reason why H2O might bond more strongly to Fe3+ than to Fe2+
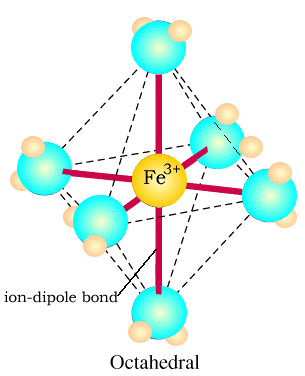
The bonds
within the water molecule are polar covalent.
Water molecules bond more strongly to Fe3+ than to Fe2+
because of the greater charge on the ion leading to a greater electrostatic
force of attraction.
Hide the solution
Hide solution
Transition metals can form coloured compounds. Cobalt, iron and copper. Zinc is a transition metal but does not form coloured compounds. The reason for this is that all its 3d orbitals are full.
All transition
metals can form complex ions.
Zn(H2O)6+2, Fe(H2O)6+2,
Cu(H2O)6+2, CoCl4-2.
Group 1 and 2 elements are metals that can form ions with only one oxidation state. In this case it is Na.
Group 1 and 2 metals can form basic oxides that are not coloured. In this case it is Na.
Solution
Most transition metals form coloured compounds. Suggest a reason why this is so.
Explain why zinc does not form coloured compounds.
Manganese exhibits many oxidation states. Explain why the greatest oxidation state of manganese is +7.
Give the oxidation state of
manganese in the following.
Mn
MnO
Mn2O3
Explain why calcium has only
one oxidation state while iron exists as a 3+ or 2+ ion.
Explain why the electronic
configuration given below is not found amongst the transition metals.
1s2, 2s2, 2p6, 3s2, 3p6,
3d4, 4s2
Give the likely electronic configuration
Solution
Hide
solution
Transition metals have electrons in a "d" subshell. Since
the "d" subshell can accomodate up to ten electrons we have
a series of ten transtion metals.
One series for the 3d another for the 4d and so on.
Nitrogen, sodium, cobalt,
iron, copper, zinc
Which of the elements above:
-can form coloured compounds;
-can form complex ions;
-is a metal and has only one oxidation state when it reacts to form different compounds.
-can form a basic oxide that is not coloured.
Which one of the following
is least likely to form a ligand with Fe3+ ions?
NH3, NH4+, H2O, OH-
Solution
Hide solution
Polar molecules, such as NH3 and H2O, can be
attracted to the Fe3+ ion and form ligands via ion-dipole
bonding.
Negative ions, such as OH- can also form ligands with Fe3+ via ion-ion bonding
Positive ions are unlikely to form ligands as there will be no electrostatic force of attraction.