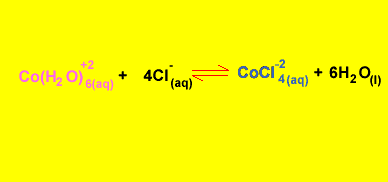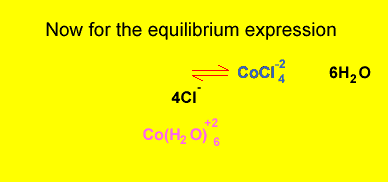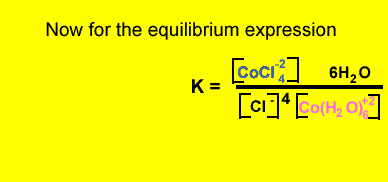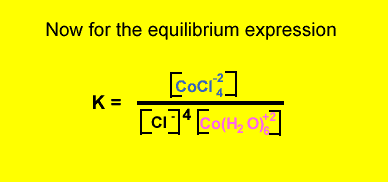
Click to hide the solution
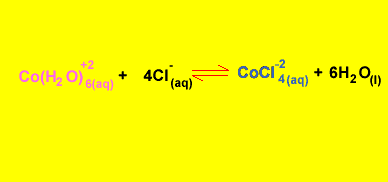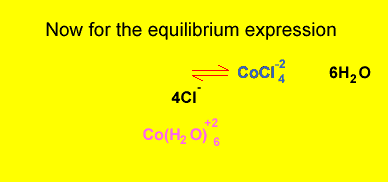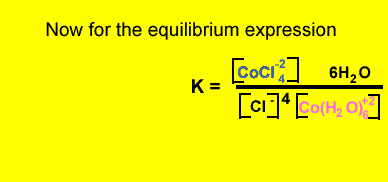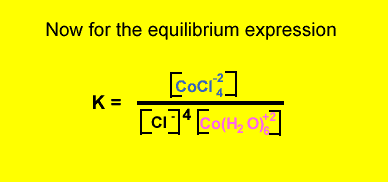
Click to hide the solution
|
Transition metal complexes
|
||
|
During this experiment we will see a range of transition metals formed by copper and cobalt ions. |
||
|
Complexes of cobalt
|
.jpg) |
|
 |
Now add 3ml of saturated NaCl solution and heat the mixture over a bunsen flame. Most of the ions are now CoCl4-2 . Note the colour change. Click to see 120kb movie. of the colour change. Now cool the mixture under running water and once again notice the colour change.
|
|
|
What is the equilibrium expression for the reaction involving CoCl4-2 and Co(H2O)6+2 ? Solution
|
||