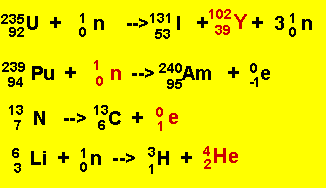
Click to hide the solutions
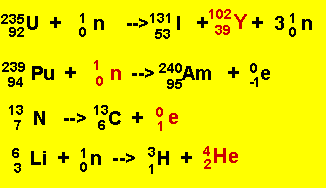
Click to hide the solutions
|
Nuclear equations |
|
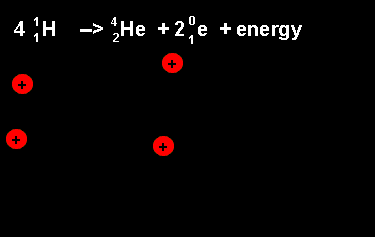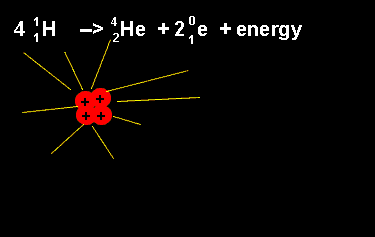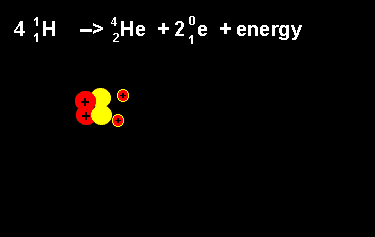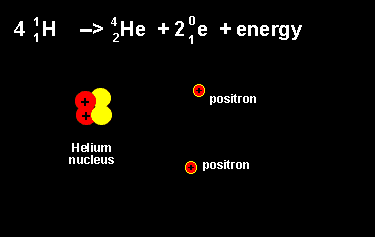 |
|
The equation above outlines the formation of a helium nucleus. The number of nucleons remains the same on both sides. When four protons fuse a helium nucleus is formed, energy given off and 2 positrons ejected. The ejection of two positrons turns two protons into neutrons. The animation on the left shows this reaction. |
|
| The animation on the right shows the nuclear fission reaction of lithium. The lithium nucleus absorbs a neutron and splits into a hydrogen and helium nucleus(alpha particle). Once again note that the number of nucleons is the same on both sides of the equation. | 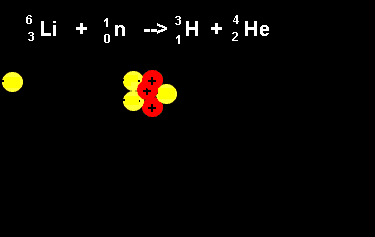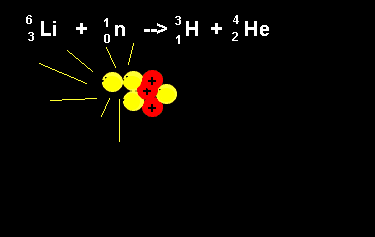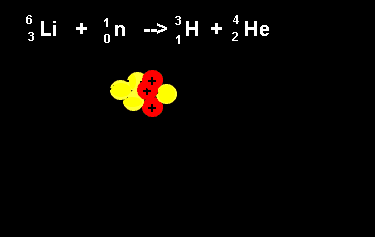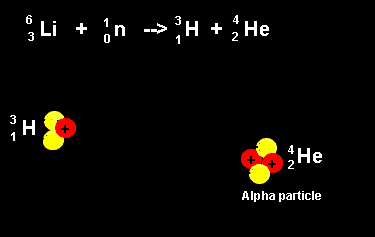 |
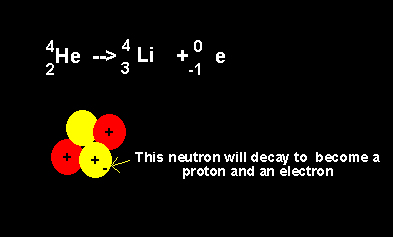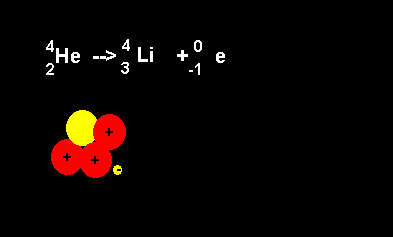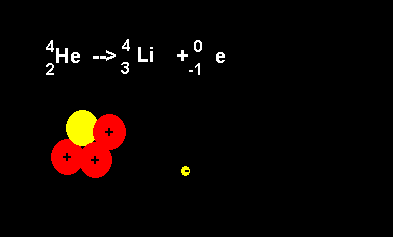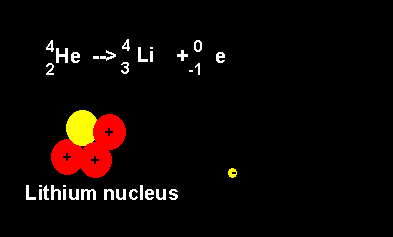 |
Helium decays to form a lithium nucleus with the release of an electron. Notice the number of nucleons is the same on both sides of the equation. A neutron decays with the release of an electron to form a proton. The atomic number increases from 2 to 3 but the atomic mass number remains the same at 4 nucleons. |
|
In summary: -a positron is emitted when
a proton decays to a neutron
|
|
|
Identify "X" in the following nuclear reactions.
Solution |
|
|
Continue
with more exercises. |
|