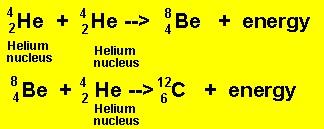The creation of elements

The sun is the birth place of all our elements. Nuclear fusion reactions fuel the giant furnace we call the Sun.
The sun was formed from a giant cloud of hydrogen which collapsed due to gravitational attraction. This contraction causes the temperature to rise to a few million degrees. At such temperatures the hydrogen atoms lose their electrons and form plasma. This plasma is a mixture of protons and electrons. As the gravitational collapse continues the temperature rises to over 10 million degrees. At this point protons have enough kinetic energy to evercome the electrostatic repulsion and nuclear fusion to form helium according the reaction below occurs.
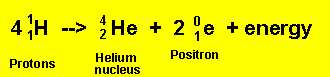

The graph above shows the mass loss per nucleon versus atomic number. As seen, iron has the most mass loss per nucleon and therefore forms the most stable nucleus. Heavier elements than iron are formed when a star implodes, providing the necessary energy needed to fuse nuclei together. Fusion reactions producing nuclei lighter than iron or iron give off energy.
In stars with a mass five times that of our own sun further contraction occurs which raises the temperature even higher to facilitate fusion between helium nuclei to form heavier nuclei.