The atom
Sodium losing electrons
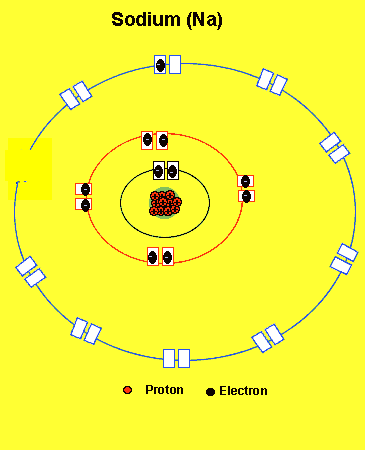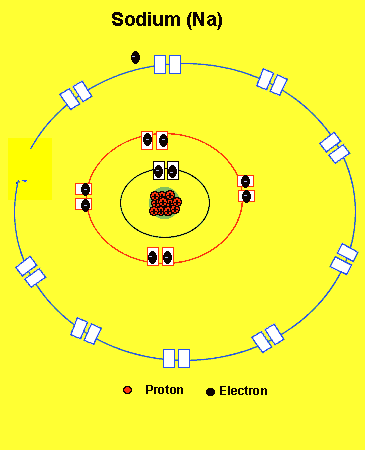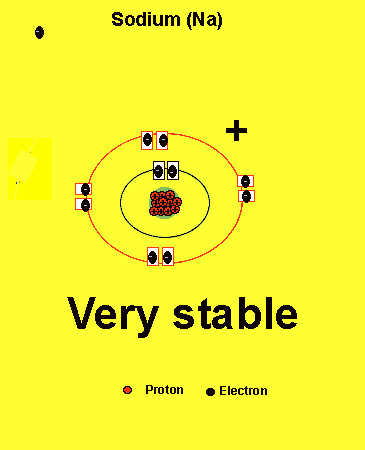
Sodium needs to lose one electron from the outer energy level.
Notice how the sodium atom loses one electron to look like neon. We can tell that it is a sodium atom by the number of protons in its nucleus. The imbalance of protons and electrons now creates a charge of +1 on the atom.
Atoms with a charge are known as IONS. More precisely atoms with a positive charge are known as cations while atoms with a negative charge are known as anions. Sodium, above, forms an cation.