The atom and its electrons
We will take a simple look at how electrons occupy the atom. Consider that all atoms have the same template. All atoms have a nucleus and energy levels in which electrons can move around. Each energy level has a certain number of spaces to accomodate electrons. The first energy level for example has 2 places, the second has 8 and the third has 18. We will only look at the first two energy levels in detail with only a brief visit to the third level.
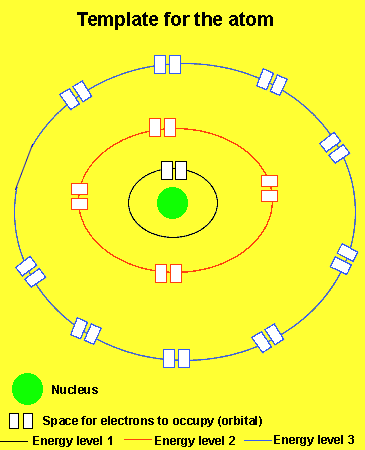
Nature starts to fill the atom from the lowest energy level first. The first energy level fills before the second. Nature also puts as many electrons in the atom as there are protons to maintain neutrality. But as we will soon see this situation does not always remain so.