The atom
In the early 1900s the atom was thought to be an indestructible ball of positive and negative charges all tightly bound together. It was a New Zealander, Ernest Rutherford that discovered the nucleus of the atom and contributed greatly to our understanding of modern day atomic structure.
Rutherford's experiment consisted of firing positive helium nuclei at a gold leaf almost 2,000 atoms thick. To his surprise the alpha particles passed straight through as though nothing was in the way. Occasionally an alpha particle would be deflected off its course and even almost bounce straight back. As Rutherford said later "It was like firing a 16" shell at a tissue paper and having it bounce back at you."
From his experiments, Rutherford concluded that protons (positive charge) must be located in a tiny, central region he called the nucleus. Most of the volume of an atom is composed of empty space with electrons moving through it.
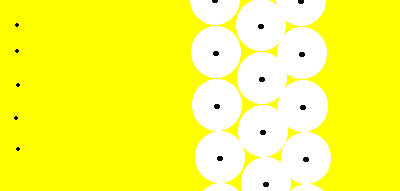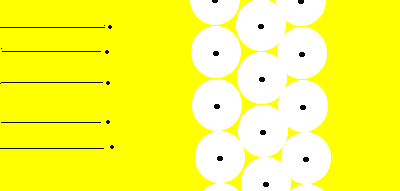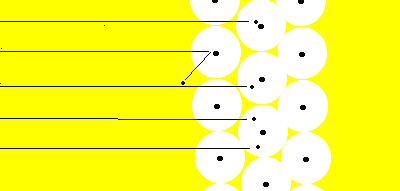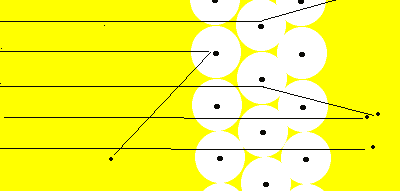
Most of the alpha particles fired at the gold foil passed straight through. Only the alpha particles that were fired straight at the nucleus were deflected from their course.
Rutherford contributed significantly to our present day understanding of the atom. Although we can not see them, we imagine atoms as fuzzy balls of negative charge surrounding a small solid nucleus composed of positive (protons) and neutral (neutrons) particles tightly packed together.
A helium atom is shown on the left

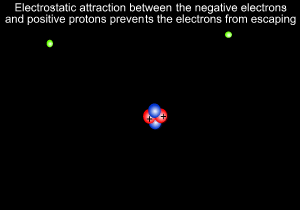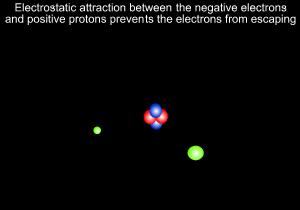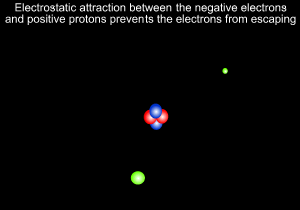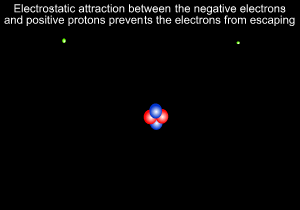
Neils Bohr, shown on the right, further refined Rutherford's model and predicted that electrons revolve around the nucleus at certain fixed orbits of particular energies.

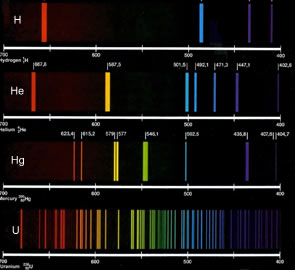
Heating an element can cause electrons to absorb energy and jump to a higher energy level. The electron returns to its original state by releasing the energy as light of a particular wavelength.
Neil's Bohr model explained the reason why emission spectra, shown on the right, are obtained when elements are heated.
Click to see the flame test of copper.
1) The nucleus of an atom consists of a
2) The charge of an electron is while the charge of a proton is and the neutron is
3) The neutrons are
4) The nucleus of an atom is
5) Which comment is true?
6) An electron
7) A neutral atom has
8) Electrostatic forces occur between and particles and are responsible for keeping the
9) An atom is identified by the number of
11) When observed from the outside an atom is described as a fuzzy ball of negative charge.
This is due to
We now know a great deal more about the atom. The proton, neutron and electron are no longer the fundamental building blocks of the atom. They are composed of even smaller units that are currently under investigation. Click to find out more