An emission spectrum is generated by the release of energy of valence electrons moving from an excited state to ground state.
The emission spectrum generated by a particular element is unique to that element and can be used in forensic investigations to identify the element present.
The emission spectra of some elements are shown below.
| Hydrogen | |
| Helium | |
| Carbon | |
| Sulfur | |
| Copper | |
| Iron |
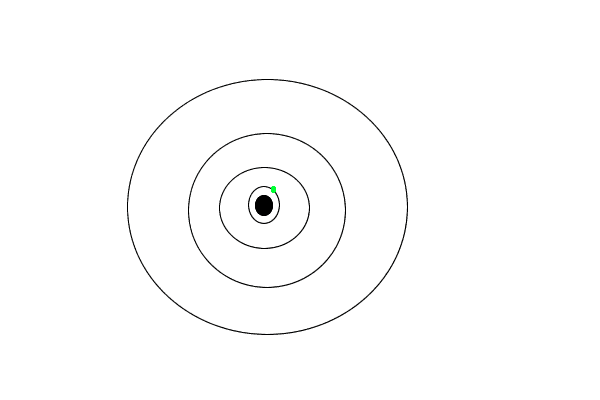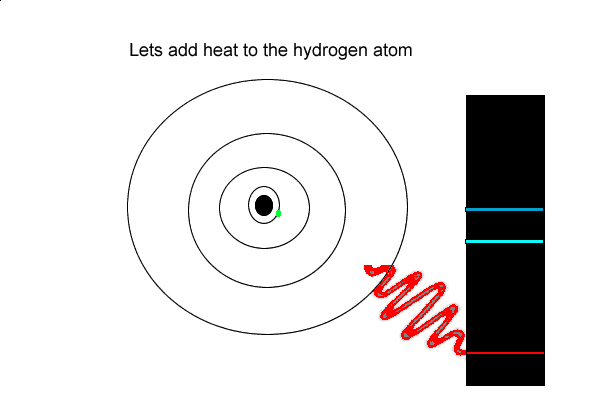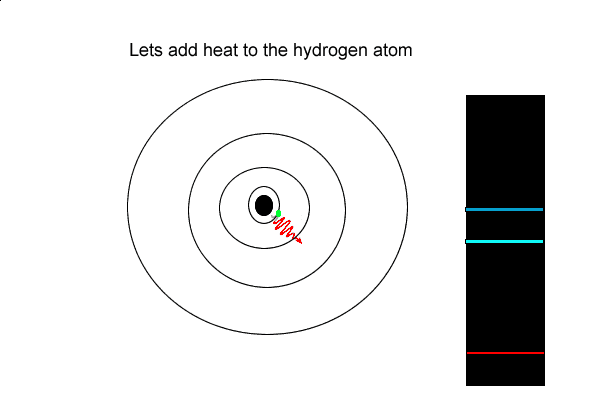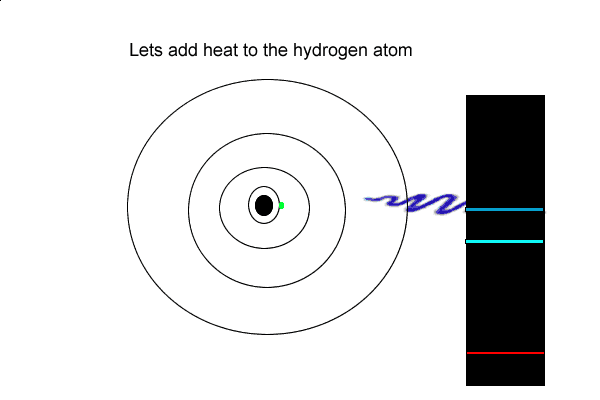
View the video on the left
1) An electron can absorb
2) When an electron absorbs energy it
3) An electron that has absorbed energy and move to a higher energy state is most likely to
4) Explain how dark lines appear in the spectrum of white light passing through a cloud of hydrogen gas.
5) How is an emission and an absorption spectrum similar?
6) Using the hydrogen emission spectrum explain how an emission spectrum is formed.
7) Deep ultra violet light
8) A photon of red light involves
9) Looking at the diagram below, which statement is true?
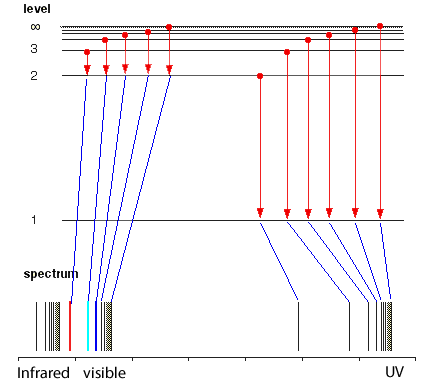
But the hydrogen electron doesn't only have the electron jumps that cause spectral lines in the visible region but also in the UV and infrared regions.
As you can see from the diagram on the left, a number of jumps are possible
If an electron falls from the 3-level to the 2-level, red light is seen. This is the origin of the red line in the hydrogen spectrum. By measuring the frequency of the red light, you can work out its energy. That energy must be exactly the same as the energy gap between the 3-level and the 2-level in the hydrogen atom.
View other emission spectra