Spectroscopy practice questions
To answer the following questions, consider the data and 1H NMR spectrum on the right
The mass spectrum of this compound shows a molecular ion at m/z=113, the IR spectrum has characteristic absorptions at 1200, 1700 and 1735 cm-1, and the 13C NMR spectrum has five signals. The absorption at 1735 cm-1 is due to a carbon atom triple bonded to a nitrogen atom.
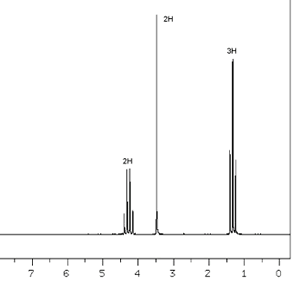
Question 1
How many types of nonequivalent protons are there in this molecule?
Solution
Question 2
What is the significance of the 13C NMR data?
Answer 3
Answer - The molecule has 5 chemically different carbon atoms.
Solution
Question 3
What type of bonds are present in the molecule as revealed by the IR spectrum? Consult a data sheet.
Solution

Question 4
What is a likely structure for this compound?
Solution