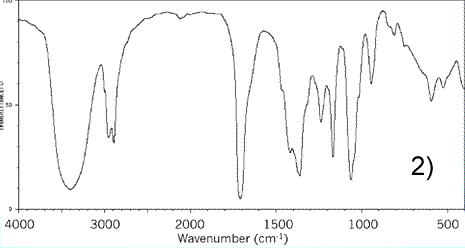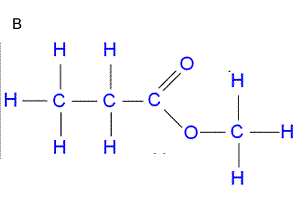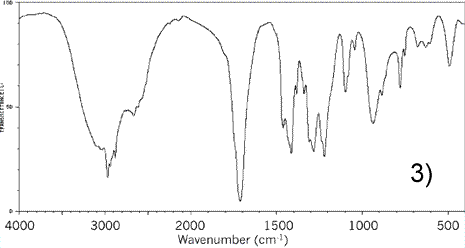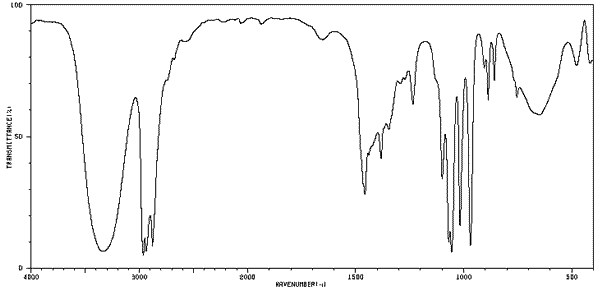
Consider the IR spectrum of propanol shown on the left.
Notice some of the key features, namely the:
-broad absorption trough at 3200 - 3550 indicating an O-H (alcohol)
-
absorption trough at about 2850 - 3000 indicating an C-H bond
-absorption trough at about 1050 - 1100 indicating a C-O bond
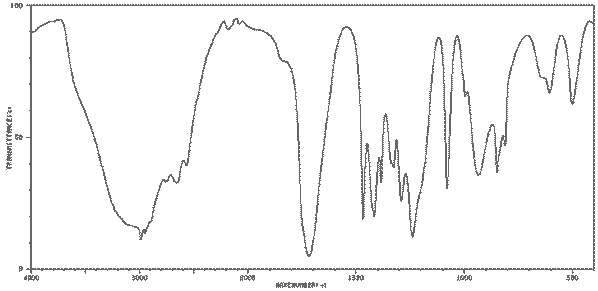
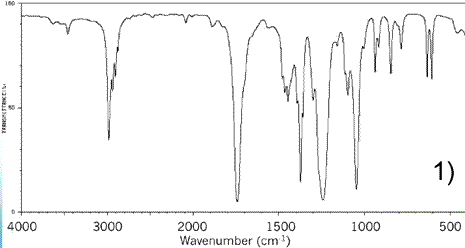
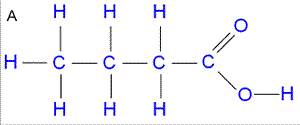
Consider the IR spectrum of propanoic acid shown on the left.
Notice some of the key features, namely the:
-broad absorption trough at 2500 - 3300 indicating an O-H (acid)
-
absorption trough at about 2850 - 3000 just poking out from under the O-H absorption trough, indicating an C-H bond
-absorption trough at about 1670 - 1750 indicating a C=O bond
-
absorption trough at about 1300 indicating a C-O bond