Instrumental analysis
Chromatography
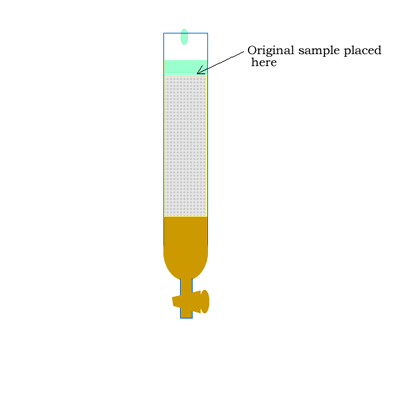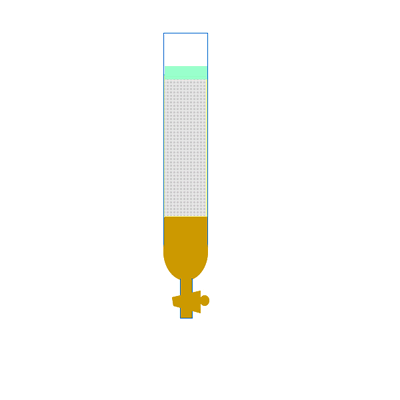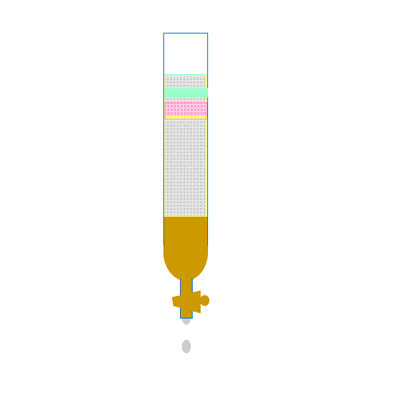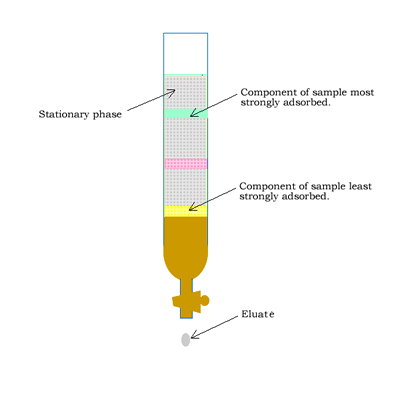
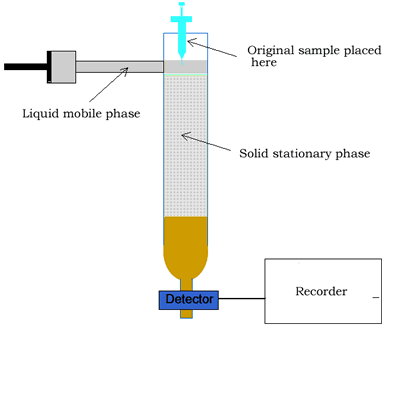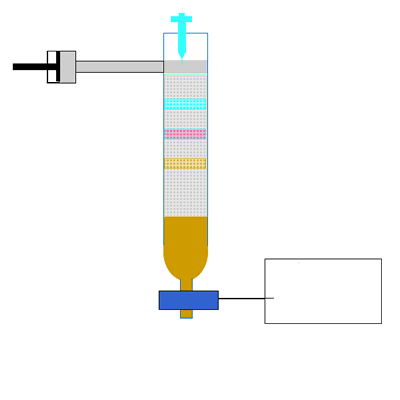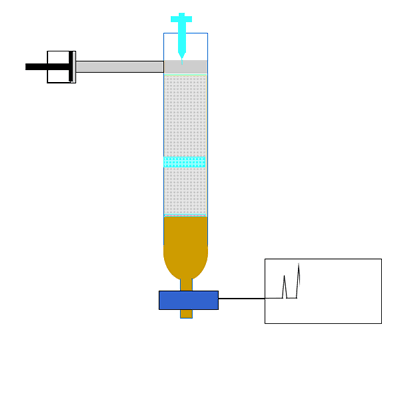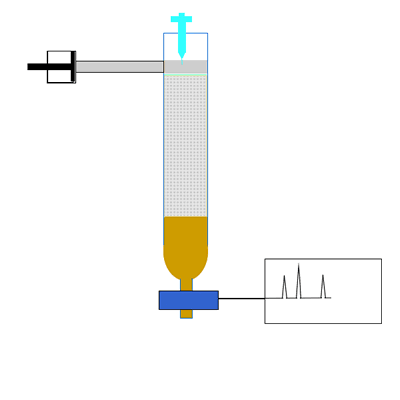
Column chromatography involves a solid stationary phase that is thinly coated with a viscous liquid and packed into a glass column, as shown on the right.
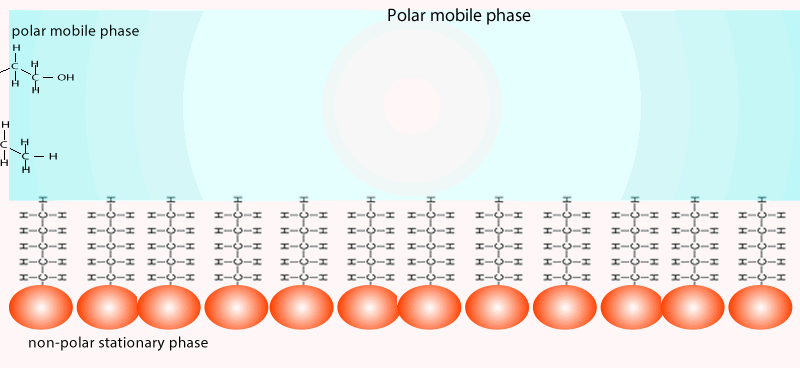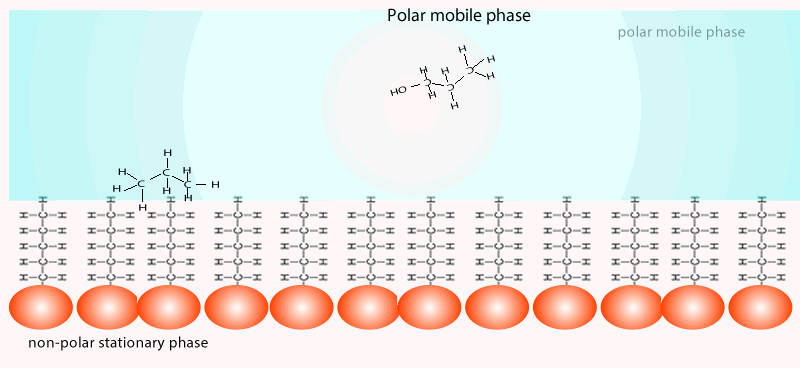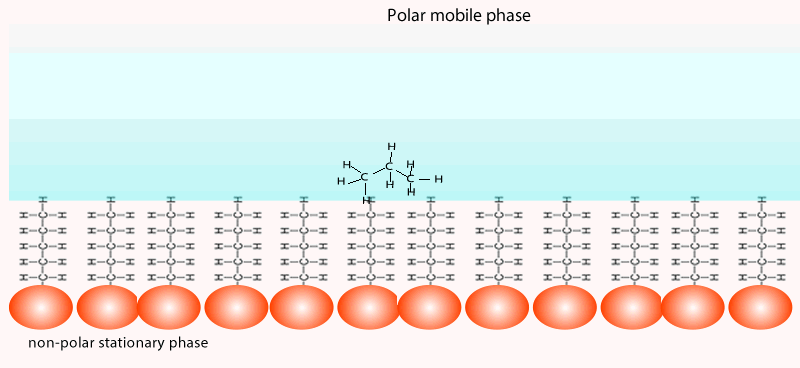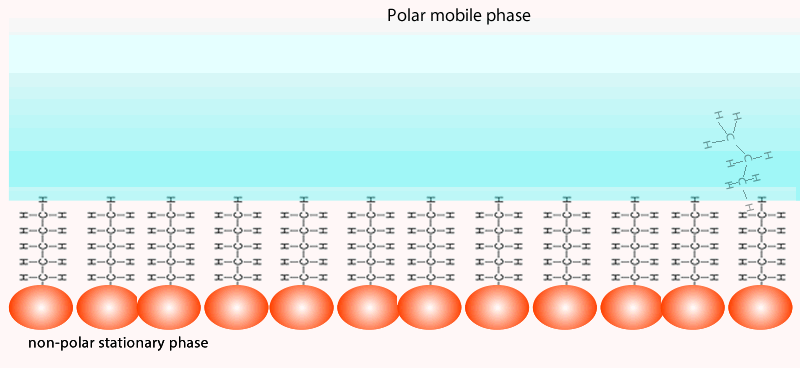
The mobile phase commonly involves a hydrophobic (non-polar) while the stationary phase is coated with a more polar compound than the mobile phase. Sometimes you will come across the term reversed-phase chromatography. The term reversed-phase describes the column setup where the mobile phase is more polar than the stationary phase, as shown in the animation below.
Along the length of the column the components of the mixture undergo a continual process of adsorption onto the solid stationary phase followed by desorption back into the liquid mobile phase. Click to see an animation.
The rate at which the different
components travel through the column depends on two factors:
- the strength with which the components adhere to the stationary phase.
- the degree to which each component dissolves in the mobile phase.
Both of the factors above depend on the unique chemical nature of the compound. As you can see from the animation below propan-1-ol is more soluble in the mobile polar phase than propane so it flows with greater speed through the column than propane. Propane interacts more with the non-polar stationary phase than the polar mobile phase.
At the end of the column a tap allows the solvent with only selected components of the mixture (eluate) to leave the
column at the same rate as the eluent enters the top of the column.
A more sensitive version of column chromatography is known as High Pressure Liquid Chromatography (HPLC). This method is widely used by hospitals to detect chemical compounds in the blood.
HPLC differs in a number of ways from the traditional column chromatography mentioned above.
1) The solid phase is made from smaller particles which significantly
increases the surface area which enables the components of the mixture
to undergo more frequent adsorption and desorption. This gives a better
separation of the components.
2) The small size of the solid particles reduces the rate of flow of the
solvent and therefore high pressure, of around 13,000 KPa, is used to
drive the solvent through the column.
The components of a mixture are detected by passing the eluent through UV light. Organic compounds absorb UV light, so the amount of UV light that falls on the detecting device is directly proportional to the quantity of organic substance. Other detectors include attaching a mass spectrometer to the column to accurately identify the substance in the eluent. The amount of UV light is recorded as a chromatogram. A chromatogram is a series of peaks that correspond to the different components in the mixture. The area under each peak is a measure of the relative amount of a particular component.
Compounds are identified by comparing the retention time(Rt). This is the time it takes for a substance to pass through the column. A compound is identified by comparing the retention time of each component with the retention times of known compounds.
sourced form https://www.youtube.com/watch?v=qXmSb6Xwr5k 10.51 7/08/20