13C is a naturally occurring isotope of carbon that has a nuclear spin. As a consequence it is used in NMR spectroscopy to identify different environments in which carbon atoms are bonded within a molecule.
13C NMR spectra will form triplets and singlets just like H NMR. However this gives a complex spectra which is hard to read. To improve the spectra allowing easy counting of the number of carbons, the protons are often irradiated at a frequency that excites prottons and gives singlets. This is known as a "proton decoupled" spectrum. Proton decoupled 13 C-NMR is probably the most widely used technique because it clarifies the spectrum making it easier to determine the number of carbon atoms.
For simplicity we will only deal with proton decoupled 13C NMR spectra.
Also keep in mind that unlike H NMR, where the area under the peaks is an indication of the number of hydrogen atoms, this is not the case in
13C NMR spectra.
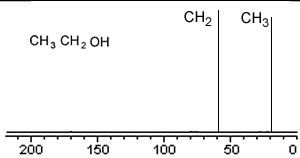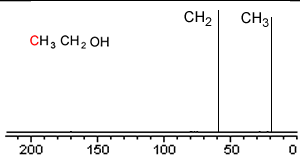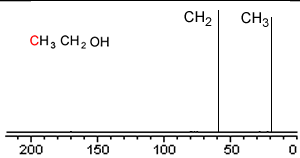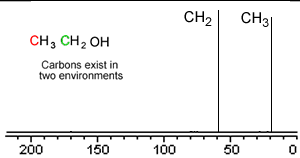
Take a simple molecule such as ethanol. It has two carbon atoms that exist in two different environments, they are shown below. The 13C NMR spectrum will therefore have two peaks.
If you recall from 1H NMR the chemical shift of nuclei is dependent on the nuclear shielding. The nuclear shielding experienced by a nucleus depends on the electronegativity of neighbouring atoms, such as oxygen. Nuclei close to oxygen tend to be shifted further to the left of the reference point (TMS). That means that the peak at about 60 (the larger chemical shift) is due to the CH2 group because it has a more electronegative atom attached.
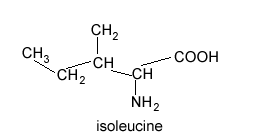
Isoleucine is shown on the right. How many peaks will be present in its 13C NMR spectrum?
If you selected 6 then your are correct. The 6 carbons exist in different environments
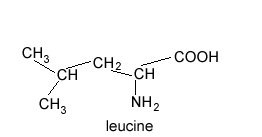
How many peaks are found in the 13C NMR spectrum of leucine?
To view the solution pass the cursor over the image on the right.
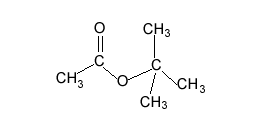
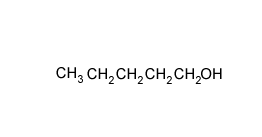
A compound has the empirical formula C4H9Br
Name and draw all the possible isomers and give the number of peaks in an 13C NMR spectrum of each isomer.
Acetone has the following semi-structural formula CH3COCH3 How many peaks will the 13C NMR have and identify to which carbon each peak belongs to.
Solution

