A burette packed with finely divided alumina powder (Al2O3) was used to separate the components in a plant extract by chromatography. The alumina acts as the stationary phase and water was used as the mobile phase.
A solution of the plant extract was placed at the top of the alumina and, once it had been adsorbed, further water was added periodically. The components separated as three coloured bands, as shown in the diagram below.
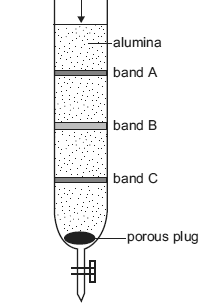
In which one of the three bands, A, B or C, were the components most strongly adsorbed to the stationary phase?
Solution
Band B starts to show signs of separating into two different bands just before it emerges from the bottom of the burette. Give one possible modification that could be made to this experiment to more effectively separate band B into its separate components.

In another experiment, the components in the plant extract were separated in a similar way to paper
chromatography, using a glass sheet coated with alumina and water as the mobile phase. Which one of the three bands, A, B or C, contains the component that would be most likely to have the smallest Rf value?
Explain your answer.
Solution
Which one of the three bands, A, B or C, contains the component that would have the smallest retention time if this separation was conducted using a high-performance liquid chromatograph with alumina as the stationary phase? Explain your answer.
Solution
