Sodium is one of the most reactive metals that reacts vigorously with water to release heat and produce hydrogen gas. The reaction releases so much energy that the hydrogen gas ignites.
View the video of sodium reacting in water. It is unlikely that you will find pure sodium in nature. Iron is reactive but not as reactive as sodium.
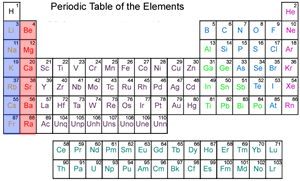
The table on the right shows the different metals in order of reactivity with the most reactive on top.
Click on the names of the metals to see where they appear on the periodic table below.
Gold been the least reactive of the metals shown can be found in its elemental form in nature.
Potassium |
Sodium |
Calcium |
Magnesium |
Aluminium |
Zinc |
Iron |
Lead |
Copper |
Silver |
Gold |
The most reactive metals are found on the left of the periodic table, in the blue column, known as the alkali metals. Their reactivity increases as we go down column (group) one, shown in blue. Click to see how the reactivity of group one metals increases down a group.
The red column is also composed of reactive metals known as alkaline earth metals and their reactivity also increases as we move down the column.
| Iron will react slowly with other elements and this is why we dig it out from the ground as iron ore (iron oxide). It is unlikely that you will find pure iron in the ground, most would have already reacted with oxygen or other chemicals. |  |
Reactive metals, such as magnesium, are widely used in rust prevention and are known as sacrificial anodes. Iron will rust unless protected in some way. Reactive metals, when attached to less reactive metals, have the ability to prevent the less reactive metal from rusting. Pictured on the right is a magnesium anode that is connected to the iron propeller of small boats. The magnesium metal protects the iron propeller from rusting. This however accelerates the rusting of the magnesium anode and it must be replaced periodically in order to keep the propeller rust free. |
 |
| Reactive metals when placed in water will produce hydrogen gas. Hydrogen is lighter than air and was used widely, early last century, in giant air-ships. Why is it not used for this purpose today? View a 40kb of the hydrogen pop test. |  |

What metals are not very reactive? (think of what metals are found in pure form in nature)
Copper, silver and gold are metals that are found in the periodic table in the same column. Does their reactivity increase as we move down the column?
Which is more reactive francium or lithium? Explain.
Galvanising is a process that involves covering sheets of iron with zinc and is used to protect iron from rusting.
i)
Why will this protect iron from rusting?
ii) What other metals can, in theory, be used to cover iron so that it is protected from rusting?
iii) Why is zinc the only practical metal to use? (look at the video on the right of sodium in water)
Where is hydrogen widely used today?
What is formed when liquid hydrogen burns in oxygen?
Why must hydrogen and oxygen be stored in liquid form in the external fuel tank?