Determination of Vitamin C
Before you undertake this investigation you may need to read how Vitamin C acts as an anti-oxidant and its role in our body. Click to go to the article.
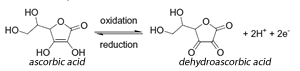
An unknown concentration of vitamin C or ascorbic acid can be determined using iodine. When ascorbic acid comes into contact with iodine, the chemical reaction that occurs produces dehydroascorbic acid and iodide. A starch indicator is added to the vitamin C solution to indicate when the chemical reaction is complete. As long as there is still ascorbic acid in the solution, the iodine will react with it and become iodide. When all the ascorbic acid has reacted and become dehydroascorbic acid, the iodine will react with the starch to create a dark blue color, signaling the reaction is complete. The dehydroascorbic acid can be measured to determine the concentration of vitamin C in the original solution.
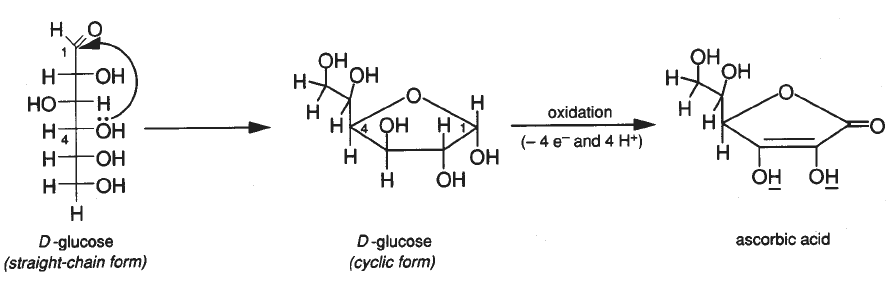
The reaction above shows the formation of ascorbic acid from the straight chain form of glucose.
3) Many fruits and vegetables react with oxygen in the air and turn brown when cut. You would have noticed this with apples. Vitamin C is often used to keep bananas and apples in a fruit salad from browning. Why?
Calculations
1) Calculate the average volume of iodine solution delivered by the burette from the concordant titres.
2) From this volume calculate the mol of I2 reacting
3) Using the stoichiometric equation below, calculate the mol of ascorbic acid present.
ascorbic acid + I2 → 2 I− + dehydroascorbic acid .
The half equation for the oxidatin of ascorbic acid to dehydroascorbic acid is shown on the right.
4) Calculate the mol of ascorbic acid per 100 grams of the food you have tested.
5) Calculate the mass of ascorbic acid in mg per 100 gram of food.
6) If the average daily allowance of ascorbic acid is 60 mg what mass of the food you have tested must be eaten every day?
7) Stephen conducted the titration and found that the average titre is 5.00 mL. He was hoping to get a titre between 15 -30 mL. What should he do?
8) The food to be tested should be prepared, ideally, only a few minutes before the titration. The reason for this is that