Climate change man made? How do we know?
Weather presenters are not trained scientists.
What arguments does the weather presenter offer to argue the case against global warming and its consequences?
He argues that we should look at the million year history of climate change and not focus on the last 100 years. What is so special about the last 100 years?
What evidence do both presenters give to dismiss climate change as a man-made phenomenon?
The weather presenter appears a little confused. He claims that we are more likely to perish from ocean acidification and lack of fresh water than global warming. Explain how increases in atmospheric carbon dioxide causes ocean acidification, and how climate change can influence the current pattern of rainfall causing a decrease in availability of fresh water.
What evidence is given that it is the sun that is causing global warming?
Click to see a graph of the solar irradiance 1880 to 2009. Use this to argue the case that the sun is, most likely, not the cause of global warming.
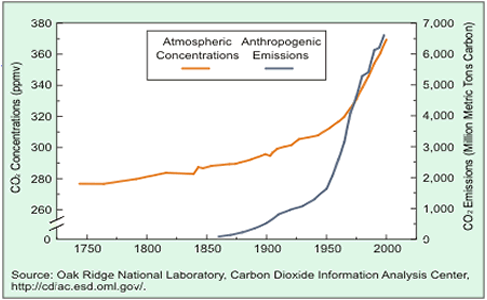
Atmospheric carbon dioxide levels have been increasing since the industrial revolution as shown on the right.
Most people do not deny that carbon dioxide is a greenhouse gas and that its levels in the atmosphere are increasing, however its origin is hotly disputed by many.
How do we know that the burning of fossil fuels like coal, oil and natural gas contribute to the increase in atmospheric carbon dioxide?
Scientists have a way of determining the origin of carbon dioxide in the atmosphere. It is a process similar to that used to carbon-date fossils.
View the video on the right.
1) What is the difference between carbon-12 and carbon-13?
2) Chemically how do carbon-12 and carbon-13 behave?
3) How is carbon-14 formed?
4) What is the half-life of carbon-14?
5) What happens to the composition of carbon in a dead animals remains over time?
Carbon is composed of three isotopes which are carbon-12, carbon-13 and carbon-14. Carbon consists mainly of the carbon-12 and carbon-13. A small amount of the carbon atom is the radioactive isotope carbon-14. In the upper atmosphere cosmic rays from the Sun react with nitrogen to create carbon-14. Carbon-14 is unstable and over time is converted back to nitrogen.
After 60,000 years there is no carbon-14 remaining in the original sample because it has been completely converted to nitrogen.
Fossil fuel reservoirs are composed of organic matter. They are buried for millions of years and over this time the carbon-13 and carbon-14 have been completely decayed leaving behind only carbon-12. Atmospheric carbon contains all three carbon isotopes (carbon-12, carbon-13 and carbon-14).
When fossil fuels are burnt only carbon-12 is released into the atmosphere as carbon dioxide. Over time, the percentage of carbon present as carbon-14 and carbon-13 should decrease as a result of burning fossil fuels.
This is exactly what is being observed. Measurements of the isotopic composition of atmospheric carbon dioxide show a steady decline of carbon-14 and carbon-13.
Explain how we know that the increase in atmospheric carbon dioxide is man made?