| Petroleum industry. | ||
Petroleum is formed from the decomposition of plant and animal matter under high pressure and heat over millions of years. It is a most useful source of raw material for the synthesis of many useful products, as will be discussed later. Products from petroleum range from plastics to food additives. View the video on the right. The arguments put forward by this presenter are commonly put forward by others who wish to promote natural products by creating concern. The presenter is obviously not a chemist. She states, about food additives made from petrochemicals, "Whether you swallow it or whether you inhale it, its petroleum and it can do a lot of harm to different people". Why is this not true? Are we eating or inhaling petroleum as this presenter suggests? Explain with reference to chemical reactions.
|
||
However this is not to say that we should except that petroleum is a safe product. On the contrary it contains many known carcinogens. Even the humble Vaseline is now rumored to have links to cancer. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1128845/ Occup Environ Med. 1997 September; 54(9): 686–691.
Go to the link above and read the study. What are the findings of this study? Benzene is a hydrocarbon that can be found in various concentrations in crude oil. It is a known carcinogen and yet one of the most useful and versatile hydrocarbons known. Benzene is a natural component of crude oil, and is an aromatic hydrocarbon (ring structure). Benzene used to be an additive in petrol, but now its use is strictly limited due to benzene been identified as a carcinogen. However, it is an important industrial solvent and raw material to the synthesis of industrial chemicals including drugs (penicillin), plastics (such as polystyrene), synthetic rubber, fragrances (Oil of Wintergreen) and dyes. Since benzene is a known carcinogen, are these drugs and plastics made from benzene dangerous? |
 |
|
Ethene is a hydrocarbon that predominantly comes from petroleum. Polyethene is a plastic synthesised from ethene and is used to produce plastic containers that are used to package food and water for human consumption. According to the argument put forward by the presenter, in the video above, since ethene comes from petroleum we are actually exposing our food and water to the many carcinogens found in petroleum (crude oil). Should we be concerned about the safety of these containers? Explain. |
 |
|
|
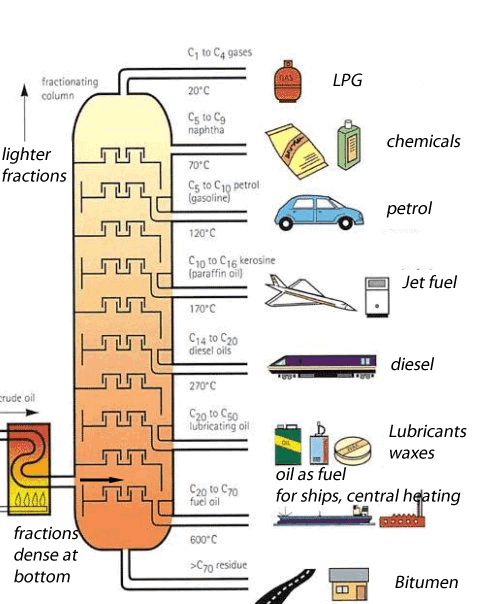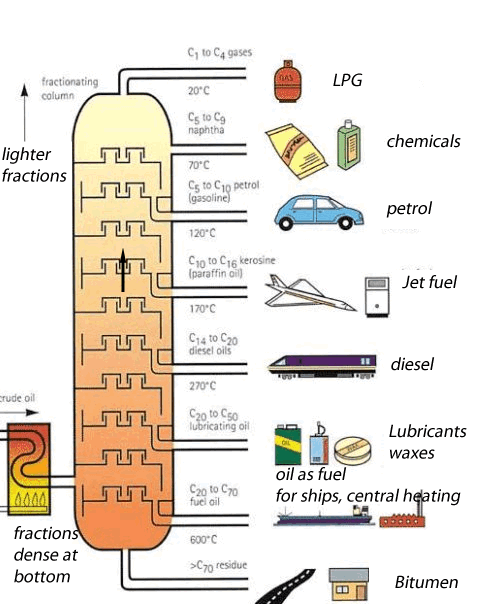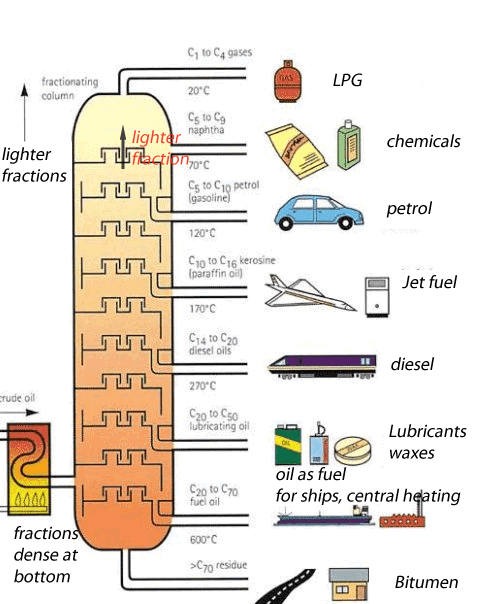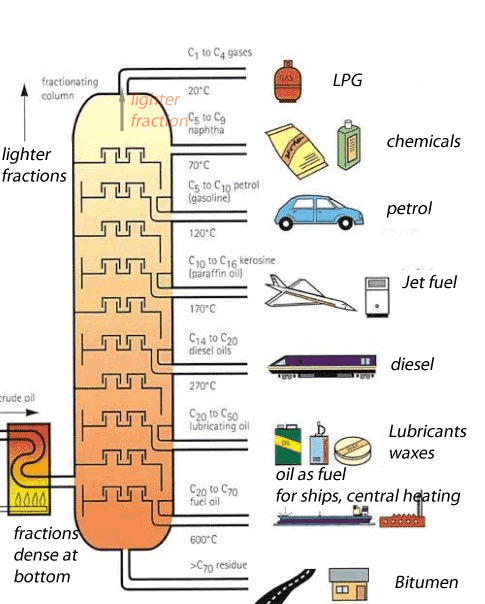
Crude oil is a mixture of hundreds of different types of hydrocarbons ranging from small molecules that form gas to long molecules that form bitumen. These molecules are composed of hydrogen and carbon , hence the name hydrocarbons. Separating the different hydrocarbons must be completed before anything useful can be obtained. The separating technique that is used in industry is called fractional distillation. This technique is shown on the left. Hydrocarbons are separated according to their boiling temperatures. The fractional distillation tower has a temperature gradient ranging from about 600 OC at the bottom to about 20 OC at the top. Different sized hydrocarbons have different boiling temperatures, the heavier ones have high boiling temperatures while the lighter ones have low boiling temperatures. This way they are separated according to their boiling temperature and collected at different points along the fractionating tower. The process by which liquids are separated by evaporation and then condensed back to liquids is known as distillation. This is what happens in an oil refinery - in one part of the process, crude oil is heated and the different molecules are pulled out by their boiling temperatures. Each different molecule has a different property that makes it useful in a different way as shown on the left |
|
| Continue with Formation of Esters | ||