Emulsifiers or surfactants
are molecules that have special properties. They contain two parts. One that can dissolve in water and one that can dissolve in oil.
Emulsifiers reduce the tendency of oil and water to separate into layers by forming an emulsion. An emulsion is formed when tiny droplets of one liquid are suspended in another liquid.
Two types of emulsions can be formed oil-in-water and water-in-oil. Oil-in water emulsions feel cool on the skin while water-in-oil feel greasy.
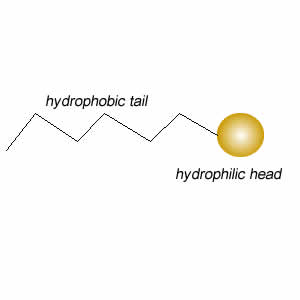
These molecules change the surface properties of liquids. The hydrophobic (water hating) or lipophilic tail (oil loving) of these molecules burrows into the oil leaving exposed the hydrophilic (water loving)or lipophobic (oil hating) head. When the oil and water mixture is agitated micelles form.
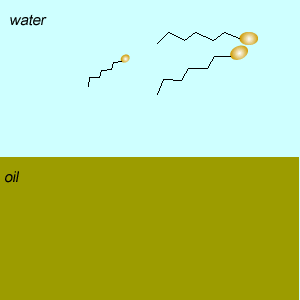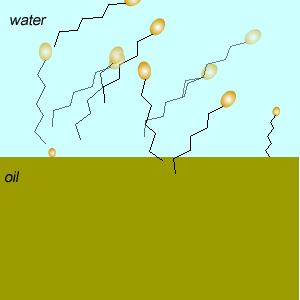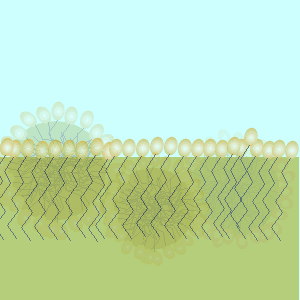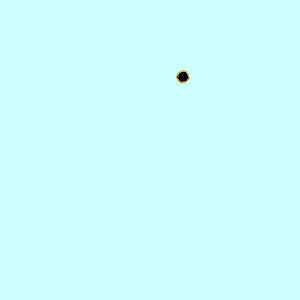
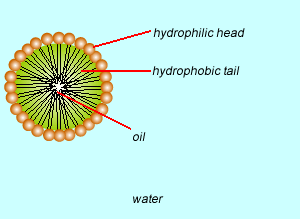

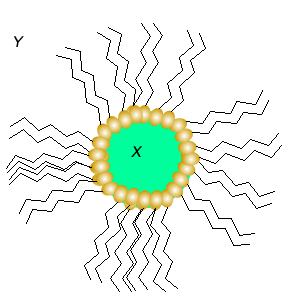
Substance X is most likely to be
Substance Y is most likely to be
This is a in emulsion.